Evolution Encyclopedia Vol. 2
Chapter 10 DNA AND PROTEIN PART 2
![]()
3 - AMINO ACIDS AND PROTEIN
PROTEIN NEEDED ALSO—NOW let's look at protein:
Putting protein and DNA together will not make them alive, but, on the other hand, there can be no life without BOTH the protein and the DNA. Proteins would also have had to be made instantly, and in the right combination and quantity—at the very beginning. And don’t forget the sequence; protein has to be in its proper sequence, just as DNA has to be in its correct sequential pattern.
Making those amino acids out of nothing, and in the correct sequence,—and doing it by chance—would just as impossible, mathematically, as a chance formation of the DNA code!
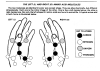
LEFT-HANDED—Twice in chapter 7 (Dating Methods) we mentioned the L and D amino acids. But that factor is also highly significant when considering the possibility that amino acids could make themselves by chance.
Nineteen of the twenty amino acids (all except glycine) come in two forms: a "D" and an "L" version. The chemicals are the same, but are arranged differently for each. The difference is quite similar to your left hand as compared with your right hand. Both are the same, yet shaped opposite to each other. Each of these two amino acid types are called "enantiomers" [en-anti-AWmers]. (Two other names for them are enantiomorphs and sterioisomers).
For simplicity's sake, we will in this study call them the left amino acid (the "L") and the right amino acid (the "D").
CLICK TO ENLARGE
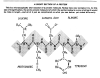
A SHORT SECTION OF A PROTEIN
This is a microscopically short section of a protein molecule. Notice how very complex it is. At this size of magnification, the entire protein molecule (of which the section below is a very small part) would be miles in length—and all of it just as complicated as what you see below, yet coded differently the 20 left amino acids. —And all those amino acids must be left-handed, not right-handed!
(For purposes of simplification we will assume that right-handed amino acids never occur in living amino acids, but there are a few rare exceptions, such as in the cell walls of some bacteria and in some antibiotic compounds.)
We will discuss the origin of sugars later in this chapter. Brown summarizes the "handedness" of both amino acids and sugars:
"Each type of amino acid, when found in nonliving material or when synthesized in the laboratory, comes in two forms that are chemically equivalent. About half of these amino acids can be described as 'right-handed' and the other half as 'left-handed.' Each structure is a mirror image of the other. However, the amino acids that comprise the proteins found in living things, including plants, animals, bacteria, molds, and even viruses, are essentially all left-handed. No known natural process can isolate either the left- or the right-handed variety. The mathematical probability that chance processes could produce just one tiny protein molecule with only left-handed amino acids is virtually zero.
"A similar observation can be made concerning sugars which, in living organisms, are essentially all right-handed . . Based on our present understanding, natural processes produce equal proportions of left- and right-handed sugars. Since the sugars in living things are almost all right-handed, our present understanding leads to the conclusion that natural processes probably did not produce life."—Walter T. Brown, In the Beginning (1989), p. 8.
THE LEFT (L)AND RIGHT (D) AMINO ACID MOLECULES
CLICK TO ENLARGE
"Many researchers have attempted to find plausible natural conditions under which L-amino acids would preferentially accumulate over their D-counterparts, but all such attempts have failed. Until this crucial problem is solved, no one can say that we have found a naturalistic explanation for the origin of life. Instead, these isomer preferences point to biochemical creation."—Dean H. Kenyon, affidavit presented to U.S. Supreme Court, No. 85-1513, in "Brief of Appellants," prepared under the direction of William J. Guste, Jr., Attorney General of the State of Louisiana, October 1985, p. A-23.
TOTAL IGNORANCE—AS we shall see in a forthcoming section of this chapter (Originating DNA), scientists have a fairly good idea of the multitude of chemical steps in putting together a DNA molecule, but not only can DNA not be synthesized "by nature" at the seashore, highly-trained technicians cannot do it in their million-dollar laboratories!
"The evolution of the genetic machinery is the step for which there are no laboratory models; hence we can speculate endlessly, unfettered by inconvenient facts."—*R. Dickerson, "Chemical Evolution and the Origin of Life, " in Scientific American, September 1978, p. 70.
Dozens of inherent and related factors are involved. One of these is the gene-protein link. This had to occur before DNA could be usable, yet no one has any idea how it can be made now, much less originate by random accident in a mud puddle.
"None has ever been recreated in the laboratory, and the evidence supporting them all [being produced by random chance in the primitive environment] is very thin. The emergence of the gene-protein link, an absolutely vital stage on the way up from lifeless atoms to ourselves; is still shrouded in almost complete mystery. —*A. Scott, "Update on Genesis," in New Scientist, May 2, 1985, p. 30.
For additional information see quotation supplement, "5 - DNA, Protein, and the Cell, " at the end of this chapter.
4 - SYNTHESIZED PROTEIN
THE MILLER EXPERIMENTS—In 1953, a graduate biochemistry student (*Stanley Miller) sparked a non-oxygen mixture of gases for a week and produced some microscopic traces of nonliving amino acids. The headlines of the world screamed the news: LIFE HAS BEEN CREATED! Some amino acids had been synthetically produced, but at no time were they alive. Dead matter had not been endued with life.
Scientists had hoped for a breakthrough and believed that this was the beginning of it. But then tragedy struck. The researchers examined the non-living amino acids which they had produced—and discovered they were composed of equal amounts of left and right amino acids!
In spite of the newspaper headlines, the scientists already knew they had not "created life," and now they discovered there was no possible way their synthetic amino acids could be useful in producing living tissue.
Because they were composed of both left and right amino acids, researchers could not make those amino acids into living creatures, nor could living creatures even use them as food!
The newspaper headlines should have bannered the words: CHANCE CREATION OF LIFE DISPROVED!
Whenever they are synthetically made, amino acids always contain equal amounts of both the left and right forms. Yet only the LEFT amino acids are ever found in living creatures! Interspersed left and right amino acids would mean instant death to any creature having them in its body.
"Amino acids synthesized in the laboratory are a mixture of the right-and left-handed forms, and thermodynamically the two forms are indistinguishable." —*Harold Blum, Time's Arrow and Evolution (1968), p. 159.
Although they seem so much alike (they are mirror images of each other), only the left-handed forms are made by or usable to living creatures.
Add to this problem the fact that it is not even practically possible to separate the left from the right ones in a laboratory. Only by closest examination can a lab expert even distinguish between the two, and it is an extremely tedious, time-consuming task. Days of painstaking work would only separate a small microscopic quantity of left from right amino acids.
Since only the left-handed amino acids are used throughout all living tissue, artificially-made batches would be useless since they always consist of equal amounts of both left and right forms.
The outcome of all this was that the *Miller synthesis of amino acids provided yet another proof that evolution could not occur! It showed that, even IF a spark could have turned some chemicals into amino acids in that ancient "Primitive environment," those amino acids would have been composed of equal amounts of left and right forms, and the presence of the right ones would clog the machinery, and destroy its mechanism, and kill any life form they were in.
The above paragraph, alone, destroys evolutionary origin-of-life theories.
SYNTHESIZED AMINO ACIDS IMPOSSIBLE TO PRODUCE— We have examined the DNA code barrier, the protein code barrier, and the left amino acid barrier. Now let us view how the scientists make their synthetic amino acids. Here we find yet another barrier:
Evolutionists claim that nature created life by chance millions of years ago. Let us now learn some valuable lessons from scientific procedures to artificially do the same thing:
(1) Synthesizing amino acids requires very complicated equipment in the extreme! No oxygen can be present, continuous sparks must be generated against chemicals for a week or so, etc. The problem is that such laboratory conditions do not occur in nature.
(2) Special mixtures of chemicals have to be brought together. These mixtures would never be found in nature.
(3) Both left and right amino acids are produced. After synthesizing them, they cannot be separated into all left amino acids, except by the use of very intelligently-controlled, highly sophisticated lab methods. It can only be done with painstaking slowness. This would not happen in nature, and if it did, the creatures would die because of the very slowness of the operation.
(4) Of the synthesized amino acids that are produced, neither the left or right are alive. How would life be added to them? Scientists know of no way to produce life in any dead object, organic or inorganic. Neither science nor nature can make dead chemicals into living creatures.
(5) The pitifully small collection of synthesized amino acids would then need to be gathered into protein molecules of the right chemical sequence. When we discuss protein formulas, we are faced with a formidable barrier:
[1] There are 20 amino acids.
[2] There are 300 amino acids in a specialized sequence in each medium protein.
[3] There are billions upon billions of possible combinations!
[4] The right combination from among the 20 amino acids would have to be brought together in the right sequence—in order to make one usable protein properly.
(6) In addition to this, the ultra-complicated DNA strands would have to be formed, along with complex enzymes, and more and more, and still more.
Yes, each of the above six barriers is massive! There would be no way for any of it to happen in nature outside the laboratory—by chance methods, or even with the aid of intelligent laboratory assistants to help the process along.
WHAT ARE THE CHANCES?—What are the chances of accomplishing all the above—and thus making a living creature out of protein manufactured by chance from dust, water, and sparks? Not one chance in billions. It cannot happen.
Evolutionists speak of "probabilities" as though they were "possibilities," if given enough odds. But reality is different than their make-believe numbers.
There are odds against your being able to throw a rock with your arm—and land it on the other side of the moon. The chances that you could do it are about as likely as this imagined animal of the evolutionists, which makes itself out of nothing and then evolves into everybody else. A mathematician would be able to figure the odds of throwing the rock as a scientific notation with 50 or so zeros after it, but that does not mean that you could really throw a rock to the moon! Such odds are not really "probabilities," they are "impossibilities!"
The chances of getting accidentally-synthesized left amino acids for one small protein molecule is one chance in 10210. That is a number with 210 zeros after it!
Such probabilities are indeed impossibilities. The number is so vast as to be totally out of the question.
Here are some other big numbers to help you grasp the utter immensity of such gigantic numbers: Ten billion years is 108 seconds. The earth weighs 1026 ounces. From one side to the other, the universe has a diameter of 1028 inches. There are 1080 elementary particles in the universe (subatomic particles: electrons, protons, neutrons, etc.). Compare those enormously large numbers with the inconceivably larger numbers required for a chance formulation of the right mixture of amino acids, proteins, and all the rest out of totally random chance combined with raw dirt, water, and so forth.
How long would it take to walk across the 1028 inches from one side of the universe to the other side? Well, after you had done it, you would need to do it billions of times more before you would even have time to try all the possible chance combinations of putting together just ONE properly sequenced left-only amino acid protein in the right order.
The above paragraph eliminates the possibility of life forms ever having originated or evolved!
When *Stanley Miller synthesized a few microscopic amino acids in 1953, he did not know what amino acids he might get, and those he did get were not in any particular sequence of any use. It was a totally random, useless sequence. And, of course, there was no life in them.
Later on, researchers tried to synthesize proteins. But they found that the only way they could do it was to use actual left amino acids from living tissue, and then carefully put them together in the sequence they copied from actual proteins! What had they accomplished? Nothing, absolutely nothing. But this mattered not to the media; soon newspaper headlines shouted, "SCIENTISTS MAKE PROTEIN!"
"The apparatus must consist of a series of proteins as well as nucleic acids with the 'right' sequences."— *R. W. Kaplan, "The Problem of Chance in Formation of Protobionts by Random Aggregation of Macromolecule, " in Chemical Evolution, p. 320.
5 - MORE PROBLEMS WITH PROTEIN
ALL 20—BUT IN 39 FORMS—The evolutionists tell us that, at some time in the distant past, all the proteins made themselves.
There are approximately 20 different essential amino acids. Each of them, with the exception of glycine, can exist in both the L (left-handed) and D (right-handed) structural form. In living tissue, the L form is found; in laboratory synthesis, equal amounts of both the L and D forms are produced. There is no way to synthesize the L form by itself.
Here are all 39 forms. What a hodgepodge for the random accidents of evolution to sort through—and come up with only the L forms:
1 - Glycine
2a - L-Alanine 2b - D-Alanine
3a - L-Valine 3b - D-Valine
4a - L-Leucine 4b - D-Leucine
5a - L-Isoleucine 5b - D-Isoleucine
6a - L-Serine 6b - D-Serine
7a - L-Threonine 7b - D-Threonine
Sa - L-Cysteine 8b - D-Cysteine
9a - L-Cystine 9b - D-Cystine
10a - L-Methionine 10b - D-Methionine
11 a - L-Glutamic Acid 11b - D-Glutamic Acid
12a - L-Aspartic Acid 12b - D-Aspartic Acid
13a - L-Lysine 13b - D-Lysine
14a - L-Arginine 14b - D-Arginine
15a - L-Histidine 15b - D-Histidine
16a - L-Phenylalanine 16b - D-Phenylalanine
17a - L-Tyrosine 17b - D-Tyrosine
18a - L-Tryptophan 18b - D-Tryptophan
19a - L-Proline 19b - D-Proline
20a - L-Hydroxyproline 20b - D-Hydroxyproline
ONE PROTEIN CODE—For a moment, let us examine one of the twenty proteins. We will look at tryptophan synthetase A. It is just one of the millions of proteins in your body, and it has a total of 2,015 separate units.
Here are the sequence of amino acids that are in this one protein, tryptophan synthetase A. Examine it for a while and you will see that there is hardly any pattern that you can identify. —Yet that total sequence is the complete code of this one protein! Not one particle of it can be omitted without serious problems or death for the organism. Imagine trying to make this ONE amino acid by chance! Each part of it is composed of a variety of the twenty amino acids, all in the left form, and the entire protein chain in a very special and exacting sequence. If that sequence is altered in any manner, it will not function properly. Damage or death may quickly follow.
CLICK TO ENLARGE
Tryptophan synthetase A
PROTEIN INFORMATION CONTENT—An information scientist from Berkeley, *Yockey, calculated the amount of data in the least complex life. He concluded that only ONE living protein could possibly have evolved in a billion years. This conclusion is not based on the chemical difficulties of putting the protein together (we will discuss that shortly)—but only getting the information content together.
"Taking into account only the effect of the racemic mixture the longest genome which could be expected with 95% confidence in 109 corresponds to only 49 amino acid residues. This is much too short to code a living system so evolution to higher forms could not get started. geological evidence for the "warm little pond" [in which Darwin suggested that life began] is missing. . Clearly 109 years is far too short a time and the universe is far too small to select even one molecule of cytochrome c from the primitive milieu. Therefore a belief that proteins basic for life as we know it appeared spontaneously in the primitive milieu on earth is based on faith."—*H. Yockey "A Calculation of the Probability of Spontaneous Biogenesis by Information Theory," in Theoretical Biology 67 (1977), p. 377.
For additional information see the appendix topic, "6 - Amino Acid Functions, " at the end of this chapter.
6 - ORIGINATING FIVE SPECIAL MATERIALS
The next five sections really belong in the previous chapter on Primitive Environment. But we are placing it here, because you now have a better understanding of the complexities of DNA and protein:
1 - ORIGINATING THE PROTEINS—How does a protein come into existence from sand, dirt, and water? There are fantastically large problems involved in the initial formation of amino acids (the building blocks of protein). And there are serious problems with the coding built into protein. We will discuss this in the next chapter on DNA.
But now, just for a moment, let us consider some of the immense chemical hurdles required to produce proteins out of nothing:
(1) - Laboratory simulations only give low yields of amino acids. Too many tars and other products are produced.
(2) - Cross reactions would have occurred between amino acids and sugars, amines, ketones, aldehydes, and carboxylic acids. These cross reactions would prevent the amino acids from forming into proteins. Other cross reactions between proteins and formaldehyde would also ruin the proteins.
(3) - Coupling agents would be needed, and they generally operate in too unspecific a manner.
(4) - The original "primitive soup" would not have been rich enough for amino acids and protein to form. An extremely concentrated mixture of just the right chemicals would have been required.
(5) - Excess water in the mixture would quickly revert the mixture back to the originally-separated chemicals.
(6) - The temperatures required to produce the amino acid intermediates, would have destroyed the end-product proteins.
(7) - Energy must come from somewhere to activate an amino acid.
(8) - This activation by an outside energy source would increase the rapidity of chemical breakdown into its separate parts.
(9) - During this activation process, each step in amino acid formation would have to be accomplished by isolation from the outside world.
(10) - Most polypeptides and proteins that could possibly be formed would be biologically useless. This is because they would have non-proteinous amino acids among them. In laboratory experiments, this latter type is consistently four times as numerous. There are complicated biochemical reasons why certain amino acids are not usable.
(11) - Amino acids, when formed in the laboratory, tend to form beta, gamma, and epsilon peptide bonds, instead of the biologically-useful alpha peptide bond.
(12) - Only L-amino acids (left-handed ones) are biologically useful, and 50 percent of those randomly produced are D-amino acids (right-handed ones). Even one D amino acid mixed in with a host of L amino acids-would render the entire batch unfit for use in a living substance.
(13) - No method has been devised by which intelligent men could enrich an amino acid mixture with more L-amino acids—by even a 1 percent increase—at the time of amino acid synthesis, so it would be impossible in nature to produce a 100 percent L-amino acid.
(14) - All randomly-produced protein material would be biologically unfit, since their amino acids were not in the proper sequence. This sequence is very complicated, just as the DNA code is very complicated. Each of the more than 20 proteins used in living tissue has its own amino acid sequence code.
(15) - Certain protein-type compounds cannot be made even under the most careful laboratory conditions. This would include phosphorylated compounds, such as ATP, GTP, and others which are vitally essential in cells for energy transfer.
2 - ORIGINATING THE SUGARS—What is involved in mud, sand, and water producing the sugars by merest chance? The likelihood is even worse, if that is possible, than for protein to form by random action. Yet sugars are crucial to life! They would have to evolve BEFORE the genetic code could be developed! And without the genetic code, any living form produced would die in the first generation. The problem here is that sugars are a basic constituent of nucleic acids, the primary chemicals in DNA and RNA.
(1) - It has been suggested that sugars evolved out of the condensation of formaldehyde, but there would not have been enough formaldehyde in the oceans to produce them. Also, formaldehyde in the oceans or atmosphere would have been destroyed by environmental factors.
(2) - Even if some simple sugars could have formed, they would quickly have been destroyed by cross reactions with amino acids, or by decomposition from other chemicals. All sugars are quite unstable outside of living tissue. They too quickly unite with other substances and break down.
(3) - Sugars formed during formaldehyde condensation are always of the simple type (saccharides). Advanced and complex sugars (disaccharides and polysaccharides) are never so formed, and no one knows how they could have formed outside of living tissue.
(4) - Because of their instability, sugars could only have been in the very lowest of concentrations in the primitive "soup" that supposedly produced initial life forms.
(5) - Only D (right-handed) sugars are used in living tissue, and any chance formation of them would always produce a 50-50 mixture of L and D sugars. We have here a repetition of the same imponderable problem that we have with amino acids, but in reverse.
3 - ORIGINATING THE FATS-Lipids are the fatty acids. Without these oil-like substances, no life-forms could exist. Lipids are needed in membrane and cell wall formation, as well as many other functions.
(1) - Some researchers acknowledge that lipids are "extremely difficult" to synthesize; others just go ahead and admit that no one has the slightest inkling as to how it could be done.
(2) - Lipids cannot be made without the help of specific enzymes and carrier proteins.
(3) - Lipids can only be made in the presence of highly controlled energy sources.
(4) - Lipids and nucleotides are larger molecules, and larger molecules are more difficult to keep stable during their formation and immediately afterward.
(5) - Various forms could theoretically be produced, yet only certain types would be used in tissue.
(6) - All lipids are in cis formation, which means that they have all their bonds on one side of the molecule. But, in random formation, it would be more likely for trans forms to be produced. These have their bonds on both sides, are more stable, and require less energy to operate.
(7) - Lipids are quickly destroyed by cross reactions with either magnesium or calcium. And both of these elements are abundant in living tissue. For example, when fat and calcium is mixed together, they form an insoluble soap-like product that is not usable in living cells. Yet lipids, calcium, and magnesium are routinely found in living creatures! No one has figured that one out yet.
4 - ORIGINATING THE ENZYMES—No one would live long without enzymes. They make it possible for our bodies to produce chemical reactions. This is due to their catalytic ability to accelerate those reactions. Everything alive has enzymes in it.
(1) - Each enzyme has a unique function. It does one or several specific tasks. For this reason, there are many different enzymes. Each one has its own code. Therefore, each one would have to originate in a unique manner.
(2) - Each of these thousands of different enzymes would have had to originate itself from dirt and water within that first generation, or the living creature newly-made—would quickly die. There would not even be one generation! Without enzymes in place immediately, that creature could not survive a few minutes.
(3) - It is now recognized that enzymes could only be made within living tissue, and not out in the sea water or on the seashore.
(4) - No mineral or chemical compound can take the place of enzymes. Only enzymes can perform the catalytic functions necessary for life to continue moment by moment.
(5) - Enzymes are not able to make copies of themselves. They cannot duplicate themselves, or, as the scientist would say, "replicate." They must be continually produced by other substances in the organism. So the life form would not only have to have them all in place immediately, it would have to have the know-how to immediately begin manufacturing more of them!
(6) - This ability to make enzymes would somehow have to be passed on to succeeding generations.
5 - ORIGINATING DNA—No matter where it may be found in the plant and animal world, DNA is always extremely complex. Yet nothing can live without DNA in its cells, to guide in all its functions. We have already discussed the complications involved in trying to correctly originate the coding systems in each DNA package. We will now consider the sheer impossibility of chance production of the chemical structure of the DNA molecule.
-
- As with all other factors (amino acids, sugars, fats, etc.), the primitive environment would have had to generate organic molecules.
(2) - These molecules would have had to survive, without rapidly changing back into raw materials. Both of these factors are disproved by the dozen or so major problems outlined in the last chapter (Primitive Environment).
(3) - High-energy precursors of purines and pyrimidines had to be produced.
(4) - These precursors had to be made available in rich concentrations.
(5) - The result had to be the immediate production of both base pairs of DNA. These pairs had to have the same sequence and be able to immediately unite with each other.
(6) - These base pairs had to somehow separate themselves from the jumble of similar and other molecules in the environment.
(7) - In a different location, a formaldehyde concentration of above 0.01 M had to have built up. Nowhere in nature dote such a build-up now occur.
(8) - This concentrated formaldehyde had to oligomerise [change into] sugars. No one knows how this could occur, but it had to do it anyway.
(9) - The originated sugars somehow separated from everything else, and collected off by themselves, and did it in rich concentrations (three things that never happen in nature. Take a pound of sugar, pour it on the ground and flood it with water. What happens?)
(10) - Next the DNA bases and the sugars had to come together.
(11) - Somehow they must be induced into making nucleosides, thus "fixing" the sugars. But there is no way this can be done.
(12) - This connecting of bases with sugars had be done in the correct way: A certain nitrogen atom of the base had to unite with a certain carbon atom of the sugar! The mathematical likelihood this can be done is equivalent to burning a house down, throwing dirt on the smoking timbers—and it all becomes a house again!
(13) - Concentrations of phosphate had to, in some mysterious manner, collect into rich concentrations.
(14) - These phosphates had to somehow be activated (either as a linear or cyclic polyphosphate).
(15) - This charged phosphate would then have to be brought to the nucleosides—so that it could be phosphorylated.
CLICK TO ENLARGE
(16) - There are several ways to phosphorylate something, but in order to produce usable nucleoside, only the 5'-hydroxyl of the ribose could be phosphorylated. Unfortunately, under the best of laboratory conditions, the other phosphorylations often occur; this can occur with only slight differences in the amount of heating that is applied.
(17) - A suitable coupling agent had to brought into the gradually-building DNA system.
(18) - The nucleotides had to next be polymerised, but this cannot be done without a preexisting polynucleotide template (a special chemical compound to assist in the task), and this template would not have existed in nature.
(19) - It is with the greatest difficulty that, under laboratory conditions, stable helical structures—the spiral tape shape of DNA—-can be produced.
(20) - Internucleotide bonds had to next be formed, but even lab technicians find this very difficult to do.
(21) - The acid-alkaline conditions (pH) had to be just right throughout the entire operation.
(22) - The temperature had to be just right.
(23) - All reactions had to occur while hidden from ultraviolet radiations, which is found in normal sunlight. Ultra-violet light would immediately destroy the nucleic acid-like molecules.
(24) - Translation machinery, to enable the DNA to be used in the organism, had to be immediately available.
(25) - Means of immediate replication of DNA (making copies of itself) had to be instantly available.
(26) - Reproduction methods, so that the tissue could have offspring, had to be available.
FUNCTIONS OF AMINO ACIDS—We have already examined the total impossibility of amino acids producing themselves by chance, and the insurmountable problems involved in their then being able to correctly code themselves into proteins.
There are at least 20 "essential" amino acids. These are the ones that cannot be made by the body, but must be taken in from food that is eaten.
At least 27 of the amino acids have been exhaustively analyzed in order to ascertain vital functions that could not continue without them. At the end of this chapter will be found a listing of some of the reasons why each of those 27 amino acids is vitally necessary for life. You and I must have each one in large numbers in our bodies, yet they all had to be in place within our bodies to begin with—on the first day that our first parents existed. In addition, from the start, there had to be a means of continually replicating them—making new ones to replace worn-out ones.
For additional information see the appendix topic, "5 - Amino Acid Functions," at the end of this chapter.
If we cannot make 20 of those amino acids within our bodies, but must obtain them from food intake,—how could they have been in our bodies to begin with? This is another chicken and the egg problem. Our bodies cannot make them, and can only obtain them by eating foods containing them. Yet we cannot be alive without them—and without life, we cannot eat the foods containing them!
Even assuming that somehow we managed by chance to get past day one,—why does it so happen that those necessary amino acids are to be found in the available food? This is another mystery.
The requirements for life are simply overwhelming. In this chapter we have primarily considered the chance formation of but three of them: DNA, amino acids, and protein. But there are many, many other factors involved in making it possible for us to live normal lives.
7 - ADDITIONAL
MATHEMATICAL IMPOSSIBILITIES
ALL BY CHANCE—Earlier in this chapter, we said that the possible combinations of DNA were the number 4 followed by a thousand zeros. That tells us about DNA combinations; what about protein combinations?
The possible arrangements of the 20 different amino acids is 2,500,000,000,000,000,000. If evolutionary theory be true, every protein arrangement in a life form had to be worked out by chance until it worked right—first one combination and then another until one was found that worked right. But by then the organism would have been long dead, if it ever had been alive!
Once the chance arrangements had hit upon the right combination of amino acids for ONE protein—the same formula would have to somehow be repeated for the other 19 proteins. And then it would somehow have to be correctly transmitted to offspring!
This one item of how the DNA, protein, enzymes, hormones, and all the other codes in your body would be successfully transmitted from generation to generation stuns the senses of any intelligent thinker. And before that could be done, they would all have to be correctly formulated in the first place.
How could it all be figured out by chance to begin with? How could all that information be passed on by random accidents to the next generation?
THE STREAM OF LIFE—The primary protein in your red blood cells has 574 amino acids in it. Until that formula was first worked out correctly by chance, and then always passed on correctly, neither you nor your ancestors could have lived a minute, much less survived and reproduced.
Consider the red blood cell ("RBCs," the scientists call them). This is a very common item in your body. You have billions upon billions upon billions of them; this is what makes your blood red. Each RBC has an extremely complicated formula of 574-amino acids in it! Yet trillions of them have to be made just to fill the needs of your body. Each red blood cell has about 280 million molecules of hemoglobin, and it would take about 1,000 red blood cells to cover the period at the end of this sentence. (Hemoglobin is the iron-carrying protein material in RBCs, which carries oxygen from the lungs to the tissues, and carbon dioxide from the tissues to the lungs.) Both in complexity and in enormous quantity, your red blood cells are unusual. We could fill several books this size with information about your red blood cells.
Because amino acids can exist in two forms (left and right), and in different sequences, there are 10800 possible ways hemoglobin could be arranged. But only ONE arrangement would succeed in producing and maintaining life!
MAKING PROTEIN BY CHANCE—The probability of forming 124 specifically sequenced proteins of 400 amino acids each by chance, is 1 x 1064489. THAT is a BIG number!
If we put a thousand zeros on each page, it would take a 64 Page booklet just to write the number!
The probability of those 124 specifically sequenced proteins, consisting of 400 all left amino acids each, being formed by chance, if EVERY molecule in all the oceans of 103 planet earths was an amino acid, and these kept linking up in sets of 124 proteins EVERY second for 10 billion years would be 1 x 1078436. And THAT is another BIG number! That is one followed by 78,436 zeros!
As mentioned earlier, such "probabilities" are "impossibilities." They are fun for math games, but nothing more. They have nothing to do with reality. Yet such odds would have to be worked out in order to produce just 124 proteins! Without success in such odds as these, multiplied a million-fold, evolution would be totally impossible. And that paragraph destroys evolutionary theory also.
Throughout this and the previous chapter, we have only discussed the basics at the bottom of the ladder of evolution. We have, as it were, only considered the first few instances of time. But what about all the development after that?
More total impossibilities.
ENZYMES—It is only amid an Alice-in Wonderland type of dreamy-thinking that evolutionary expectations could long survive. One must continually try to ignore the intricate marvels of the created world.
"The fundamental objection to all these [evolutionary] theories is that they involve raising oneself by one's own bootstraps. You cannot make proteins without DNA, but you cannot make DNA without enzymes, which are proteins. It is a chicken and egg situation. That a suitable enzyme should have cropped up by chance, even in a long period, is implausible, considering the complexity of such molecules. And there cannot have been a long time [in which to do it]."— *G.R. Taylor, Great Evolution Mystery (1983), p. 201.
*Fred Hoyle openly and honestly recognized this in a number of his writings. He wrote in New Scientist that 2,000 different and very complex enzymes are required for a living organism to exist. And then he added that not a single one of these could be formed by random, shuffling processes in even 20 billion years! In the following article, written to astronomers, he then adds this:
"I don't know how long it is going to be before astronomers generally recognize that the arrangement of not even one among the many thousands of biopolyers [enzymes, proteins, hormones, etc.] on which life depends could have been arrived at by natural processes here on the earth. Astronomers will have a little difficulty in understanding this because they will be assured by biologists that it is not so; the biologists having been assured in their turn by others that it is not so. The ‘others’ are a group of persons [the evolutionary theoreticians] who believe, quite openly, in mathematical miracles.
"They advocate the belief that, tucked away in nature outside of normal physics, there is a law which performs miracles (provided the miracles are in the aid of biology). This curious situation sits oddly on a profession that for long has been dedicated to coming up with logical explanations . . The modern miracle workers are always found to be living in the twilight fringes of thermodynamics." —*Fred Hoyle, "The Big Bang in Astronomy," in New Scientist, November 19, 1981, pp. 521-527.
Consider the complexity of burning sugar in the body:
"Parts of the acid chain [of the DNA molecule] fold into a helix and then the whole folds into a twisted, 'higher order' structure containing an active site at which the enzyme will bind with the substance it is going to affect. These sites are normally highly specific, and will react only with particular substates. Their specificity provides rigorous control over the chemical production line, so that transformations—for example, that of glucose into carbon dioxide, water and energy occur only along well-defined ‘metabolic pathways.’ The plan or pattern for metabolic pathways is thus determined by the sequence of enzymes employed and this, in turn, is chemically coded into the cell's nucleic acid (DNA) which is, itself, built up by enzyme action."— Michael Pitman, Adam and Evolution (1984), p. 144.
But how could such an arrangement be formed by evolutionary chance?
"Dixon [a leading enzymologist] confesses that he cannot see how such a system could ever have originated spontaneously. The main difficulty is that an enzyme system does not work at all until it is complete, or nearly so. Another problem is the question of how enzymes appear without pre-existing enzymes to make them. 'The association between enzymes and life,' Dixon writes, 'is so intimate that the problem of the origin of life itself is largely that of the origin of enzymes.' "—Op. cit., pp. 144-145.
DIXON-WEBB CALCULATION In 1964 *Malcolm Dixon and *Edwin Webb, on page 667 of their standard reference work, Enzymes, mentioned to fellow scientists that in order to get the needed amino acids in close enough proximity to form a given protein molecule, a total volume of amino-acid solution equal to 1050 times the volume of our earth would be needed! That would be 1 with 50 zeros after it multiplied by the contents of a mixing bowl. And the bowl would be so large that planet earth would be in it! That is what two knowledgeable scientists say would be needed to arrive at the proper combination of amino acids to make just one protein molecule. And that is assuming the mixing bowl (times 1 with 50 zeros) was filled with amino acids to begin with! Nothing is said here about how they would initially be made.
After using the above method to obtain ONE protein molecule, what would it take to produce ONE hemoglobin (blood) molecule, which contains 574 specifically-coded amino acids?
On page 279 of their Introduction to Protein Chemistry, *S.W. Fox and *J. F. Foster explain how that would have to be done. First, large amounts of random amounts of all 20 basic types of protein molecules would be needed. In order to succeed at this, enough of the random protein molecules would be needed to fill a volume 10512 TIMES the volume of our entire known universal And all of that space would be packed in solid with protein molecules. In addition, all of them would have to contain only left-handed amino acids (which only occur 50 percent of the time in synthetic laboratory production).
Then and only then could random chance produce just the right combination for ONE hemoglobin molecule, with the proper sequence of 574 left-handed amino acids!
But there are thousands of other types of protein molecules in every living cell, and even if all of them could be assembled by chance, the cell would still not be alive.
EVOLVING UPWARD We have not even begun to consider the mutation barrier yet. For one animal to evolve into a distinctly higher kind of animal would require a tremendous number of mutational steps.
Julian Huxley, one of the foremost proponents of mutational, evolution, has estimated that it would take millions of mutational steps.
But Huxley did not mention that each step would have to be totally accurate, or total failure would result. That is the way it is with living organisms. Just a few errors out of millions of right ones would so weaken the creature that it or its offspring would soon die off.
That is part of a biological principle called "syntropy." Each organ is useless until it is perfect, and each organ must remain perfect or it weakens and ceases to function properly. Without proper functioning within that organ, the entire organism or its offspring will tend to die out.
Neo-Darwinian evolutionists (a majority of evolutionists today) have theorized that one species changes into another one by mutational changes within the DNA code. But, recognizing the astounding interconnections between the various organs, enzymes, processes, and timing of the body, one evolutionary scientist decided that it was all far too interlocked for even one change to be successfully made in the millions of units comprising the DNA formulas.
And that point is enough by itself to totally eliminate the possibility of evolution!
"Such ancient systems are extremely conservative because so many diverse later reactions have become intricately dependent on them that they are no longer free to evolve. A mutational change which might be beneficial in one way, in almost every case would be at a strong disadvantage in many other ways. When such a mutation occurred, the process of natural selection would reject it [i.e., the body would weaken or die as a result]." *R.V. Eck, Science, April 15,1966, pp. 363-364.
(Much more information on the ineffectiveness of mutational changes as a means of evolutionary change will be found in chapter 14, Mutations.)
BEYOND DNA AND PROTEIN We have focused our attention on DNA and protein sequence in this chapter. Just for a moment, let us look beyond DNA and protein to a few of the more complicated organs in the human body. As we do so, the requirements become truly fabulous. Consider the human brain, with its ten billion integrated cells in the cerebral cortex. How could all that come about by chance? Ask an expert on ductless glands to explain hormone production to you. Your head will swim. Gaze into the human eye and view how it is constructed, how it works. You who would cling to evolution as a theory that is workable; give up! give up! There is no chance! evolution is impossible!
COMPUTER SIMULATION Computers can now simulate all those immense number probabilities that we have been speaking of in this chapter! We no longer have to leave it to imagination, and glibly say, "Given enough time and given enough chances, living creatures could arise out of sea water and lightning, and pelicans could change themselves into elephants."
Instead, we can now feed all the factors into a large computer and get fairly rapid answers. Within a dramatically short time we can find out whether evolution is possible after all! Unfortunately, the evolutionists are staying away from such computer simulations; they are afraid to face the facts. Instead they spend their time discussing their dreamy ideas with one another, and writing articles about their conclusions in scientific journals.
A computer scientist who spoke at a special biology symposium in Philadelphia laid out the facts this way:
"Nowadays computers are operating within a range which is not entirely incommensurate with that dealt with in actual evolution theories. If a species breeds once a year, the number of cycles in a million years is about the same as that which one would obtain in a ten-day computation which iterates a program whose duration is a hundreth of a second . . Now we have less excuse for explaining away difficulties [via evolutionary theory] by invoking the unobservable effect of astronomical [enormously large] numbers of small variations."*M.P. Schutzenberger, Mathematical Challenges to the Neo-Darwinian Interpretation of Evolution (1967), pp. 73-75 [an address given of the Wistar Institute of Anatomy and Biology Symposium].
Schutzenberger then turned his attention to the key point that scientists admit to be the only real basis of evolution: gradual improvements in the genetic code through beneficial mutations, resulting in new and changed species:
"We believe that it is not conceivable. In fact, if we try to simulate such a situation by making changes randomly at the typographic level by letters or by blocks, the size of the unit need not matter, on computer programs, we find that we have no chance (i.e., less than 1/101000) even to see what the modified program would compute; it just jams!"
"Further, there is no chance (less than 1/101000) to see this mechanism (this single changed characteristic in the DNA) appear spontaneously and, if it did, even less [chance] for it to remain!"Ibid.
1/101000 is one with a thousand zeros after it! In contrast, one chance in a million only involves six zeros! Compare it with the almost impossible likelihood of your winning a major multimillion-dollar state lottery in the United States: That figure has been computed, and is only a relatively "tiny" number of six with six zeros after it. Evolution requires probabilities which are totally out of the realm of reality.
Scientific notation is the use of a number plus a small superscript numeral. Using it, small numbers can be written, to denote numbers which are so immense that they are both incomprehensible and can only with difficulty be written out. Thus, 8 trillion (8,000,000,000,000) would be written 8x1012, and 1 billion (1,000,000,000) would be written simply as 109. Here are a few comparisons to show you the impossible large size of such numbers:
Hairs on an average head . 2 x 106
Seconds in a year 3x107
Retirement age (from 0 to 65) in seconds 2x109
World population 5x109
Miles in a light year 6x1010
Sand grains on all shores 1022
Observed stars 1022
Water drops in all the oceans 1026
Candle power of the sun 3x1027
Electrons in the universe 1080
Yet some of the probabilities needed to produce just one small part of evolution (such as a single protein), have required a thousand zeros (101000) or more. Concluding his statement, quoted above, Schutzenberger, an evolutionist, said this:
"We believe that there is a considerable gap in the neo-Darwinian theory of evolution, and we believe this gap to be of such a nature that it cannot be bridged within the current conception of biology. "Ibid.
Another researcher, *M. Eden, in attendance at the same Wistar Institute, said that the code within the DNA molecule is actually in a structured form, like words in a language. Letters in a language are structured in a certain sequence, and only because of the sequence can they have meaning. Eden then goes on and explains that DNA, like other languages, cannot be tinkered with by random variational changes; if done, the result will always be confusion)
"No currently existing formal language can tolerate random changes in the symbol sequences which express its sentences. Meaning is invariably destroyed."*M. Eden, "Inadequacies of Neo-Darwinian Evolution as a Scientific Theory," in Op. cit., p. 11.
And yet evolutionary theory teaches that DNA and all life appeared by chance, and then evolved through random changes within the DNA!
(For more information on those special evolutionary conferences, see chapter 29 [History of Evolutionary Theory].)
IMMEDIATE COMPLETION REQUIRED Evolutionists imagine that time could solve the problem: given enough time the impossible could become possible. But time works directly against success.
"Time is no help. Biomolecules outside a living system tend to degrade with time, not build up. In most cases, a few days is all they would last. Time decomposes complex systems. If a large 'word' (a protein) or even a paragraph is generated by chance, time will operate to degrade it. The more time you allow, the less chance there is that fragmentary 'sense' will survive the chemical maelstrom of matter." Michael Pitman, Adam and Evolution (1984), p. 233.
ALL AT ONCE Everything had to come together all at once. Within a few minutes, all the various parts of the living organism had to make themselves out of sloshing, muddy water.
"The primitive RNA strands that happened to have the right backbone and the right nucleotidase . . were simultaneously both the source of instruction (through the basepairing rules) and the target molecules to be synthesized according to that instruction. Here at the molecular level are the roots of the old puzzle about the chicken or the egg. Which came first, function or information? As we shall show, neither one could precede the other; they had to evolve together."*J. Maynard Smith, "The Origin of Genetic Information," in Evolution Now, (1982) pp. 10, 13.
"In the most part, however, conventional Darwinian theory rationalizes most adaptations by assuming that sufficient time has transpired during evolution for natural selection to provide us with all the biological adaptations we see on earth today, but in reality the adaptive process must by necessity occur rather quickly (in one or at the most two breeding generations)."*E. Steele, Somatic Selection and Adaptive Evolution (2nd ed. 1981), p. 3.
But simultaneous self-creations of as few as two factors would be more absolutely impossible than the spontaneous generation of one.
"So the simultaneous formation of two or more molecules of any given enzyme purely by chance is fantastically improbable,"*W. Thorpe "Reductionism in Biology," in Studies in the Philosophy of Biology (1974), p. 117.
"To form a polypeptide chain of a protein containing one hundred amino acids represents a choice of one out of 10130 possibilities. Here again, there is no evidence suggesting that one sequence is more stable than another, energetically. The total number of hydrogen atoms in the universe is only 1078. That the probability of forming one of these polypeptide chains by change is unimaginably small; within the boundary of conditions of time and space we are considering it is effectively zero."*E. Ambrose, The Nature and Origin of the Biological World (1982), p. 135.
PUTTING A BACTERIA TOGETHER Let us go beyond DNA molecules and pieces of protein, and consider one of the simplest of life forms. Scientists have studied in detail the bacterium, Escherichia coli, a bacteria commonly found in the large bowel.
Under favorable conditions bacterial cells can divide every 20 minutes, and, theoretically, each cell can produce 1020 cells in one day! Yet for over a century researchers have studied bacteria and have never found one to change into anything else; each one remains a bacterium. And they do not divide simply. The single chromosome replicates (makes a copy of itself), and then splits in two. Then each daughter cell splits in two, forming the various cells in the bacterium. These tiny bacteria can divide either sexually or asexually.
Escherichia coli has about 5,000 genes in its single chromosome strand. This is the equivalent of a million three-letter codons. Yet this tiny bacterium is one of the "simplest" living creatures that exists.
FRAME SHIFTS Then scientists discovered an even "simpler" creature that lives in the human bowel. It is called the theta-x-174, and is a tiny virus. It is so small, that it does not contain enough DNA information to produce the proteins in its membrane! How can it do it then? How can it produce proteins without enough DNA code to produce proteins! Scientists were totally baffled upon making this discovery. Then they discovered the high-tech secret: The answer is but another example of a super-intelligent Creator. The researchers found that this tiny mindless creature routinely codes for that protein thousands of times a day and does it by "frame-shift."
To try to describe it in simple words, a gene is read off from the first DNA base to produce a protein. Then the same message is read again but this time omitting the first base and starting with the second. This produces a different protein. And on and on it goes. Try writing messages in this way, and you will begin to see how utterly complicated it is: "Try writing messages/ writing messages in / messages in this / in this way." And THAT is how the simplest of viruses uses its DNA coding to make its protein!
Does someone think that the virus was smart enough to figure out that complicated procedure with its own brains? Or will someone suggest that it all "just happened by chance?"
With all this in mind, *Wally Gilbert, a Nobel prizewinning molecular biologist, said that bacteria and viruses have a more complicated DNA code-reading system than the "higher forms of life."
For additional information see the appendix topic, "4 - Gregor Mendel's Monumental Discovery, " at the end of this chapter.
THE CENTRAL DOGMA *Francis Crick, the co-discoverer of the structure of DNA, prepared a genetic principle which he entitled, "The Central Dogma:"
"The transfer of information from nucleic acid to nucleic acid, or from nucleic acid to protein may be possible, but transfer from protein to protein, or from protein to nucleic acid is impossible. *Francis Crick, "Central Dogma, " quoted in "Richard Milner, Encyclopedia of Evolution (1990), p. 77.
The Central Dogma is an important scientific principle and means this: The complex coding within the DNA in the cell nucleus decides the traits for the organism. But what is in the body and what happens to the body, cannot affect the DNA coding. What this means is this: Species cannot change from one into another! All the members in a species (dogs, for example) can only be the outcome of the wide range of "gene pool" data in the DNA, but no member of that species can, because of the environment or what has happened to that individual, change into another species. Only changes in the DNA coding can produce such changes; nothing else can do it.
"It has proved a fruitful principle, ever since James Watson and Crick discovered the double-helix structure of DNA in the 1950s. DNA is the blueprint; it gives instructions to the RNA and to proteins about how to arrange themselves."*Richard Milner, Encyclopedia of Evolution (1990), Ibid.
CONCLUSION How long would it take for lifeless chemicals under ideal conditions (concentrated mixtures, just the right amount of incoming energy, no oxygen in the atmosphere changing suddenly into oxygen, no ultra violet rays in sunlight, etc.) to turn themselves by random chance into a single protozoan? All the chemicals had to be there in the proper relationship, but, of course, that would still not make it alive.
A mathematician, William Feller,
figured that it would be equivalent to setting a monkey down before a typewriter
and keeping him at the task of typing random strokes at an incredibly fast 12
keystrokes per second for practically forever, and, eventually, pounding
irrationally at his typewriter, turning out the words in Genesis 1:1, complete
and in the proper order. Here is Feller's conclusions, as written for us by
Bolton Davidheiser:
LIFE CYCLE OF THE THETA-X-174 VIRUS
CLICK TO ENLARGE
"Try to think of a rock so large that if the earth were at its center, its surface would touch the nearest star. This star is so far away that light from it takes more than 4 years to get here, traveling 186,000 miles every second.
"If a bird came once every million years and removed an amount equivalent to the finest grain of sand, four such rocks would be worn away before the champion super simians would be expected to type Genesis 1:1."Bolton Davidheiser, Evolution aid Christian Faith (1969), p. 363 [using the calculations of William Feller, in his Introduction to Probability Theory and Its Implications (1950), p. 266. Italics his.]
It is a striking fact that, left to itself, nature will always yield randomness and disorder; it is only intelligence that can produce coded order, and it is only intelligence that can replicate it.
"There is simply no way of explaining how a uniform rate of evolution could have occurred in any family of homologous proteins by either chance or selection; and, even if we could advance an explanation for a particular protein family, we would still be left with the mystifying problem of explaining why other protein families should have evolved at different rates." *Michael Denton, Evolution: Theory in Crisis (1986), p. 305.
"An honest man, armed with all the knowledge available to us now, could only state that in some sense, the origin of life appears at the moment to be almost a miracle, so many are the conditions which would have had to have been ratified to get it going."*Francis Crick, Life Itself (1981), p. 88 [co-discoverer of the DNA helix].
It took many years by carefully trained research scientists, using very expensive equipment, just to unravel the code within the DNA. If so much intelligence was needed just to discover that code, how much intelligence was needed to create it in the first place?
For any thinking person who will honestly consider them, the facts in this chapter on DNA, protein, and probabilities are enough to crack evolution to pieces, stamp on it, crush it to powder, and blow it away.
All the things you are--were wonderfully planned. You are special; do not forget that. You are special to the One who made you.
"My substance [literally, my frame] was not hid from Thee, when I was made in secret [within my mother's womb], and curiously wrought [literally, embroidered; recall to mind the intricate double-helical structure of the DNA molecule, which designs the rest of the body] in the lowest [least seen] parts of the earth [man is made of the dust of the earth].
"Thine eyes did see my-substance-yet-being-unperfect [all one word in the Hebrew, means embryo. Note that the embryo is not imperfect, but unperfect; i.e., still in the process of being completed]; and in Thy book all my members were written, which in continuance [literally, which days; all the days of development and growth were planned from the beginning] were fashioned [same word as formed in Genesis 2:7 when Adam was formed from materials already in existence] when as yet there was none of them [the entire most marvelous process was prewritten into the genetic code]." Psalm 139:15-16, plus bracketed comments.
You have just completed
Chapter 10 DNA AND PROTEIN PART 2